2025 How to Navigate API Manufacturing for Efficient Production Processes
The landscape of pharmaceutical production is rapidly evolving, with a particular emphasis on the significance of Active Pharmaceutical Ingredient (API) manufacturing. As the demand for efficient and high-quality drug production continues to rise, understanding the intricacies of API manufacturing becomes increasingly vital for industry stakeholders. The complexities involved in the sourcing, production, and quality control of APIs necessitate a comprehensive approach that encompasses best practices and innovative strategies to streamline operations.
In 2025, navigating the challenges of API manufacturing will require not only technological advancements but also a keen awareness of regulatory requirements and market dynamics. Companies must prioritize optimizing their production processes to ensure that they can meet the ever-growing consumer demand while adhering to stringent guidelines. This entails a thorough analysis of various factors, including supply chain management, production methodologies, and the integration of cutting-edge technologies that enhance efficiency and productivity.
By effectively harnessing the potential of API manufacturing, organizations can transform their production capabilities, reduce lead times, and ultimately deliver higher value to their consumers. As we explore the pathways to efficient API manufacturing, it becomes clear that a proactive and informed approach will be essential for success in this competitive landscape.

Understanding API Manufacturing: Overview of Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients (APIs) are vital components in the pharmaceutical industry, playing a critical role in the development of effective medications. The API market has been witnessing a noteworthy expansion, with a projected growth rate of 6.4% CAGR from 2022 to 2030, according to a report by Grand View Research. This growth reflects an increasing demand for high-quality, efficacious drugs and highlights the need for manufacturers to optimize their production processes to enhance efficiency and reduce costs. Understanding the intricacies of API manufacturing is essential for any stakeholder aiming to thrive in this competitive environment.
To streamline API manufacturing, companies can adopt several best practices. First, investing in advanced technologies, such as Continuous Manufacturing (CM), can lead to significant improvements in production efficiency and quality control. The FDA has recognized CM as a method that can reduce production times and decrease operating costs while maintaining compliance with stringent regulations. Second, focusing on process optimization through techniques like Quality by Design (QbD) can help in anticipating potential issues during the production phase, thus ensuring a more seamless manufacturing process.
Tips: Implementing a robust supplier risk management process is critical. Assessing and onboarding reliable suppliers can mitigate risks associated with supply chain disruptions. Additionally, conducting regular audits can ensure compliance with industry standards and improve product quality. By leveraging these strategies, API manufacturers can position themselves for success in an evolving market landscape.
Current Trends in API Production: Market Insights and Growth Projections
The evolving landscape of Active Pharmaceutical Ingredient (API) production is marked by significant trends that are shaping the market dynamics and influencing growth projections. As the demand for pharmaceuticals continues to rise, manufacturers are focusing on enhancing their production processes to ensure efficiency and compliance with stringent regulatory standards. The shift towards automation and digitalization in API manufacturing is not only optimizing production lines but also reducing costs and lead times, making processes more agile and responsive to market needs.
Moreover, the increasing emphasis on sustainable practices is driving innovation within the industry. Many companies are adopting green chemistry principles to minimize environmental impact while improving production scalability. This trend is supported by regulatory incentives that encourage sustainable practices, indicating a growing recognition of environmental responsibility among pharmaceutical manufacturers. Additionally, the rise of personalized medicine is prompting a reevaluation of API production strategies, as smaller batches and tailored formulations become more common, necessitating a flexible approach to manufacturing that meets diverse customer requirements.
Collectively, these trends illustrate a vibrant API production market poised for growth. With advancements in technology, combined with a commitment to sustainability and adaptability, manufacturers are well-positioned to navigate the complexities of API manufacturing, ensuring they meet the evolving expectations of the pharmaceutical landscape. As we approach 2025, proactive adaptation to these trends will be crucial for companies aiming to thrive in this competitive environment.
Key Technologies in API Manufacturing: Automation and Process Optimization
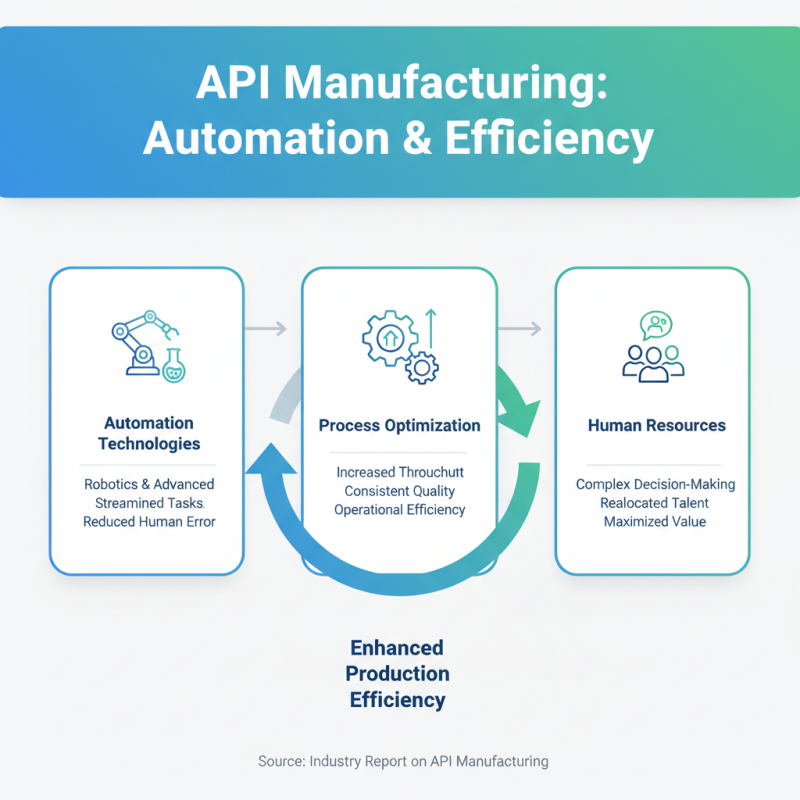
In the realm of Active Pharmaceutical Ingredient (API) manufacturing, the integration of automation and process optimization has become essential for enhancing production efficiency. Automation technologies, such as robotics and advanced process control systems, streamline repetitive tasks, reduce human error, and increase throughput. By employing automated systems, manufacturers can achieve consistent quality in their output while reallocating human resources to more complex decision-making roles, thereby maximizing operational efficiency.
Process optimization complements automation by utilizing data analytics and process modeling to refine production workflows. Techniques such as Continuous Manufacturing (CM) and Real-Time Process Analytical Technology (PAT) allow for constant monitoring and adjustment of processes, leading to minimized waste and energy consumption. By leveraging these technologies, API manufacturers can not only improve yield and reduce batch time but also ensure compliance with stringent regulatory standards. The strategic combination of automation and process optimization positions manufacturers to be more agile, responsive to market demands, and capable of consistently delivering high-quality products in a competitive landscape.
Quality Control and Regulatory Compliance in API Production Processes
In the realm of Active Pharmaceutical Ingredient (API) manufacturing, stringent quality control and adherence to regulatory compliance are paramount for successful production processes. Ensuring every batch meets the specified quality standards requires comprehensive testing and validation at multiple stages of production. From raw material inspection to in-process controls and final product testing, each step plays a crucial role in guaranteeing the safety and efficacy of the pharmaceutical products. Implementing robust quality management systems and utilizing state-of-the-art technology can significantly enhance these processes, allowing manufacturers to efficiently detect discrepancies and rectify them before they escalate.
Regulatory compliance in API production is not just about adhering to governmental guidelines; it also involves aligning with industry best practices and maintaining transparency with stakeholders. Manufacturers must stay informed about evolving regulations and standards to ensure their practices meet or exceed requirements. Frequent audits and inspections are essential in fostering a culture of compliance. Moreover, training personnel on regulatory expectations and quality standards can bolster a manufacturer's commitment to producing high-quality APIs. This proactive approach not only mitigates risks but also fosters trust among consumers and regulatory bodies alike, ultimately contributing to the sustainable growth of the manufacturing entity.
2025 How to Navigate API Manufacturing for Efficient Production Processes - Quality Control and Regulatory Compliance in API Production Processes
| Parameter | Description | Regulatory Requirement | Quality Control Measures |
|---|---|---|---|
| Batch Size | Quantity produced in a single run | Compliance with ICH Q7A | In-process checks at defined intervals |
| Raw Material Quality | Standards for sourcing APIs | FDA and EMA guidelines | Quality audits and certificate verification |
| Process Validation | Evidence that processes yield consistent results | GMP compliance requirement | Validation studies - PQ, OQ, IQ |
| Documentation | Records of production and quality control | Requirement by regulatory agencies | Batch production records and logs |
| Environmental Controls | Control of production environment | ISO 14644 cleanroom standards | Air quality monitoring and filtration systems |
Sustainability in API Manufacturing: Strategies for Reducing Environmental Impact
Sustainability in Active Pharmaceutical Ingredient (API) manufacturing has become increasingly critical as the industry seeks to meet both regulatory requirements and public expectations for environmental stewardship. According to a report from the International Pharmaceutical Federation, the pharmaceutical sector contributes approximately 4% of global greenhouse gas emissions. This statistic underscores the urgent need for strategies that minimize the environmental footprint of API production. By adopting green chemistry principles, manufacturers can reduce waste and lessen energy consumption, ultimately leading to more sustainable practices. For instance, implementing solvent-free reactions and utilizing renewable feedstocks can decrease the reliance on hazardous materials, thereby enhancing both economic and environmental outcomes.
Moreover, enhancing energy efficiency within manufacturing processes can significantly lower carbon emissions. The U.S. Department of Energy highlights that energy efficiency improvements in the chemical sector could reduce energy consumption by up to 20%, yielding substantial cost savings and minimizing environmental impacts. Additionally, integrating renewable energy sources—such as solar or wind—into API manufacturing facilities not only helps in reducing carbon footprints but also contributes to a stable energy supply in an era of fluctuating fuel prices. With regulatory frameworks and public pressures pushing for greener operations, API manufacturers that proactively incorporate sustainability strategies are likely to benefit from improved public perception and long-term viability in a competitive market landscape.
Sustainability in API Manufacturing: Strategies for Reducing Environmental Impact
This chart illustrates the percentage of different strategies adopted by API manufacturers to reduce their environmental impact in 2025. The data highlights the focus areas including waste reduction, energy efficiency, water conservation, and sustainable sourcing.
Related Posts
-

The Ultimate Guide to API Manufacturing: Unlocking Efficiency in Pharmaceutical Production
-

Maximizing Efficiency in API Manufacturing with Digital Strategies and Key Industry Insights
-

Unlocking the Advantages of API Manufacturing for Enhanced Product Quality
-

How to Optimize API Pharmaceutical Development for Maximum Efficiency
-

How to Leverage API Manufacturing to Enhance Production Efficiency by 30%: A Guide
-

The Future of API Manufacturing Innovations Driving Global Supply Chain Efficiency
